If there were any living stem cells in donated amniotic tissues to be processed for orthopedic use in another patient that would require an fda designation as a cellular drug.
Amniotic stem cell therapy fda approved.
Stem cell therapy treatment is an inpatient hospital stay.
Many pushing these amniotic and cord stem cell products claim that they have a stamp of approval from the fda so are these products fda approved.
As long as the injections are fda approved medicare covers treatments.
If you are considering stem cell treatments check to make sure the product you are considering is on the fda s approved list of stem cell treatments external icon if the stem cell product is not on the approved list or if you are considering an exosome product ask the provider to show you documentation from the fda and that they have fda s.
The full list of fda approved products is here.
Inpatient therapy for stem cells is 3 4 weeks long.
Are amniotic and cord stem cells fda approved.
There are currently no fda approved amniotic stem cell therapy products.
Find out which therapies are covered under medicare and where this form of treatment.
The clinics make outrageous and unfounded health claims i e.
Some clinics also may falsely advertise that fda review and approval of the stem cell therapy is unnecessary.
In total there are a few very fda approved live stem cell products and these are only allowed for restricted use such as for bone marrow transplantation in severe end stage cancer etc.
None of the fda approved live cell products are live umbilical cord products or live amniotic or live placenta products or exosomes or amniosomes and n one of the.
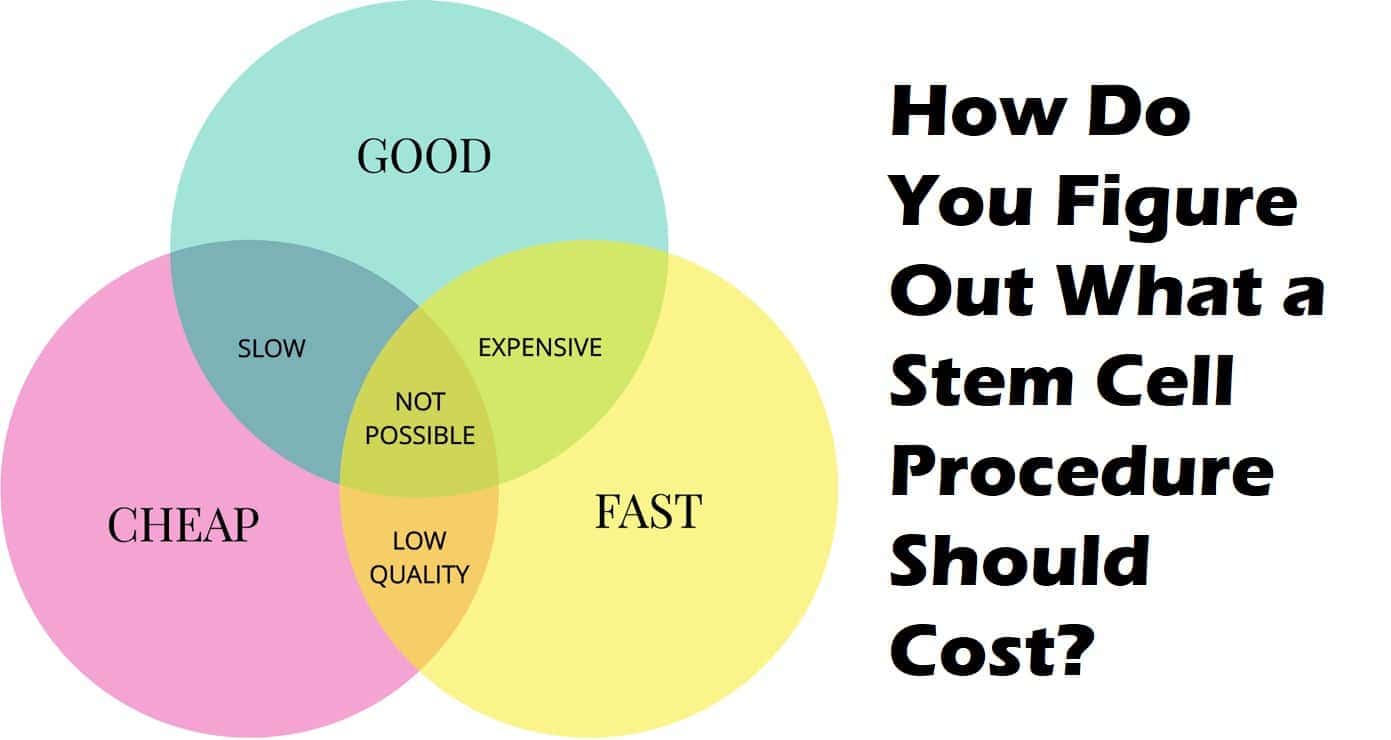
This means that the stem cell products would have to go through lengthy and very expensive clinical trials in order to potentially qualify for approval.
Stem cells from a newborn s umbilical cord blood are fda approved to treat over 80 diseases helping regenerate the body after chemotherapy radiation and other aggressive medical procedures.
Cure blindness heal all organs associated with the stem cell therapy.
Now you know that amniotic or umbilical cord cells are not fda approved.
The clinics treated patients with amniotic stem cells derived from the amniotic fluid of women who had cesarean sections.
Stem cell therapy is an approved treatment for a few specific conditions and is being studied for many more.
In the world of stem cell regulations there are two entirely different fda pathways for donor tissues.
While that s a compelling reason to bank cord blood it is important to understand what these treatments are and how stem cells from your child s.
Yet outpatient stem cell therapy will fall under part b and have a shorter duration.
In fact fda has a warning on their website about this issue.


_opt.png)





















